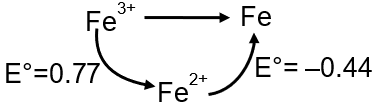
The standard reduction potential data at 25°C is given below.
E° (Fe3+, Fe2+) = +0.77 V ;
E° (Fe2+, Fe) = –0.44 V
E° (Cu2+, Cu) = +0.34 V;
E° (Cu+, Cu) = + 0.52 V
E° [O2(g) + 4H+ + 4e– → 2H2O] = +1.23 V;
E° [O2(g) + 2H2O + 4e– → 4OH¯] = +0.40 V
E° (Cr3+, Cr) = – 0.74 V;
E°(Cr2+, Cr) = –0.91 V
Match E° of the redox pair in List I with the values given in List II and select the correct answer using the code given below the lists:
| List I | List II |
| (P) E° (Fe3+, Fe) | (1) – 0.18 V |
| (Q) E° (4H2O ⇌ 4H+ + 4OH¯) | (2) – 0.4 V |
| (R) E° (Cu2+ + Cu → 2Cu+) | (3) – 0.04 V |
| (S) E° (Cr3+, Cr2+) | (4) – 0.83 V |
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Checking easiest option P or S
Cr3+ → Cr2+
E° = – 0.74 × 3 + 0.91 × 2
= – 0.4 V
Please subscribe our Youtube channel to unlock this solution.