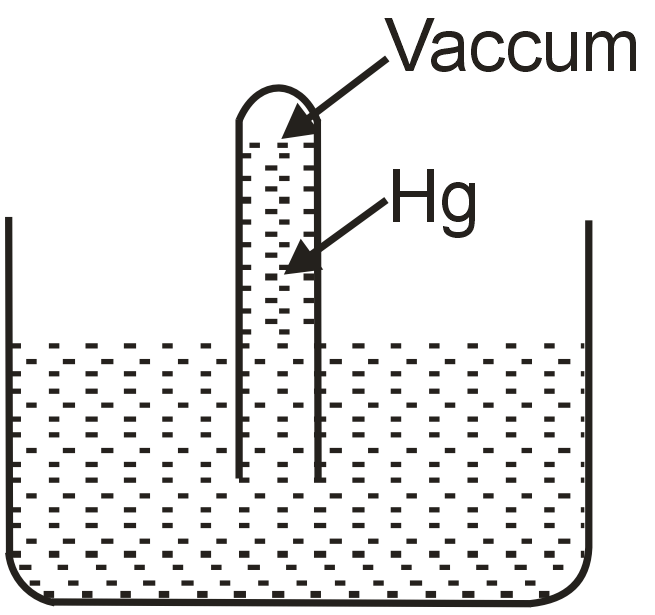
A barometer made of a very narrow tube (see fig.) is placed at normal temperature and pressure. The coefficient of volume expansion of mercury is 0.00018/°C and that of the tube is negligible. The temperature of mercury in the barometer is now raised by 1°C but the temperature of the atmosphere does not change. Then
Statement-1: The mercury height in the tube remains unchanged.
Statement-2: The atmospheric pressure remains same.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
When mercury temperature increases by 1°C, its density decreases due to volume expansion. The density ρ changes as ρ' = ρ/(1+γΔT), where γ=0.00018/°C. The barometer height h = Patm/ρg. Since Patm remains constant (Statement-2 is true) but ρ decreases, h must increase to maintain pressure balance. Thus, Statement-1 is false.
Final answer: Statement-1 is false, statement-2 is true