Engineering
Chemistry
Barometer and Manometer Pressure Measurement
Question
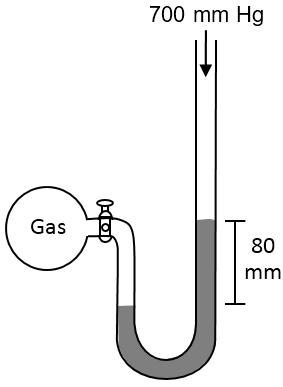
A gas is confined in the manometer as shown below. The stopcock is then opened and the highest level of mercury inside the tube moved to a level that is 80 mm above its lowest level. What is the pressure of the gas?
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
The pressure of the gas is equal to the sum of the atmospheric pressure and the pressure due to the difference in the mercury level. The pressure of the gas = 760 + 80 = 840 mm Hg.