Engineering
Chemistry
Hess Law
Question
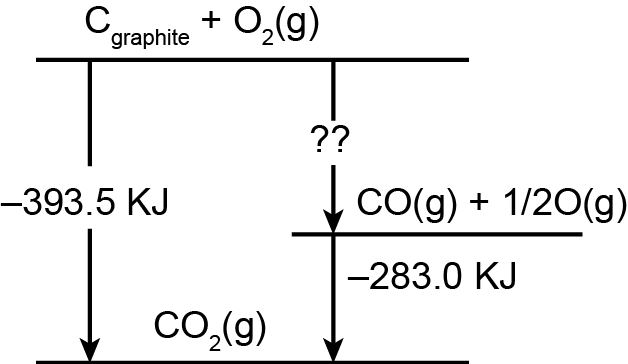
A schematic representation of enthalpy changes for the reaction, C(graphite) + O2(g) → CO(g) is given below. The missing value is
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Since enthalpy is a state function therefore it does not depend on the path . Thats y the enthalpy of direct method and indirect method of formation of product ( i . e here CO2 ) will be same so thats y.....
-393.5 = X + (-283 KJ)
X = -110.5 KJ
Hence, the correct option is C.