Amphoteric oxides, such as aluminium oxide, are soluble both in strongly acidic and in strongly basic solutions :
In acid : Aℓ2O3(s) + 6H3O+ (aq) ⇌ 2Aℓ3+ (aq) + 9 H2O (ℓ)
In base : Aℓ(OH)3(s) + OH¯ (aq) ⇌ Aℓ(OH)4¯ (aq) .........................Keq = 40
Dissolution of Al(OH)3 in excess base is just a special case of the effect of complex-ion formation on solubility. Al(OH)3 dissolves because excess OH¯ ions convert it to the soluble complex ion Al(OH)4¯ (aluminate ion). The effect of pH on the solubility of Al(OH)3 is shown in figure.
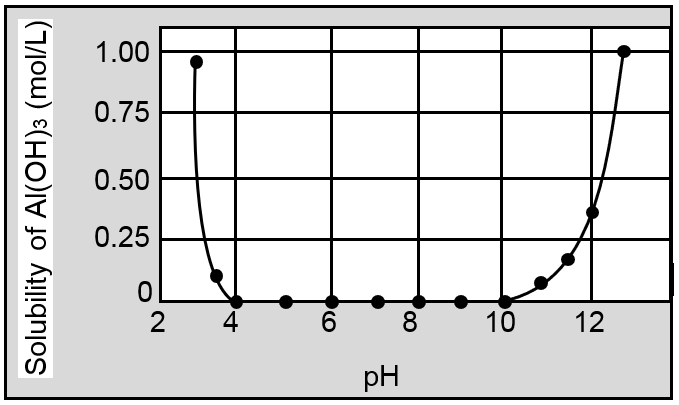
Other examples of amphoteric hydroxides include Zn(OH)2, Cr(OH)3, Sn(OH)2 and Pb(OH)2, which react with excess OH¯ ions to form the soluble complex ions Zn(OH)42– (zincate ion), Cr(OH)4¯ (chromite ion), Sn(OH)3¯ (stannite ion), and Pb(OH)3¯ (plumbite ion), respectively. By contrast, basic hydroxides, such as Mn(OH)2, Fe(OH)2,and Fe(OH)3, dissolve in strong acid but not in strong base.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Aluminium is mined as bauxite (Al2O3.xH2O) a hydrated oxide that is always contaminated with Fe2O3 and SiO2. In Bayer process, Al2O3 is purified by some reagents, which of the following reagents will be the best for this purification (Note : Strong heating of precipitate of Al(OH)3 produces Al2O3)
First heat bauxite with NaOH so, Al2O3 get dissolved in it (SiO2 will also get dissolved as it is an acidic oxide but not Fe2O3 (basic acidic),then filter lt. To filtrate add a weak acid like CO2 so Al(OH)3 forms Al3+ ions). On heating the ppt of Al(OH)3 we will get pure Al2O3
Please subscribe our Youtube channel to unlock this solution.
How many mg of solid Al(OH)3 would dissolve in 100 mL of 5M NH3(aq.) (Kb = 1.62 × 10–5).
[Mol. wt. of Al(OH)3 = 78]
[OH–] = = 9 × 10–3 M
Al(OH)3 + OH– ⇌ [Al(OH)4]–aq. K = 40
(s) aq.
40 =
∴ [Al(OH)4]– = 0.36 M
mgms of Al(OH)3 dissolved =
= 2808
Please subscribe our Youtube channel to unlock this solution.
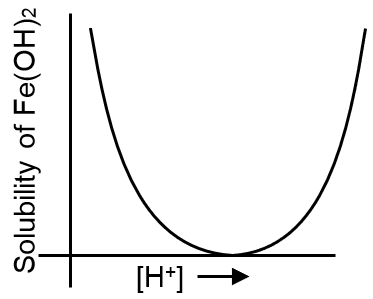
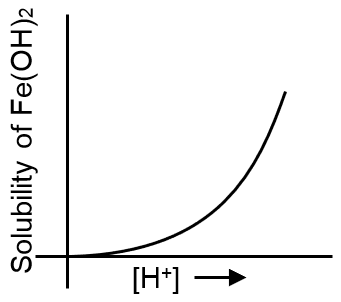
Which of the following curves best represents the variation of solubility of ferrous hydroxide Fe(OH)2 with the concentration of [H+] ions in the solution :
On increasing concentration of [H+] ions the solubility of basic hydroxide Fe(OH)2, will increase.
Please subscribe our Youtube channel to unlock this solution.