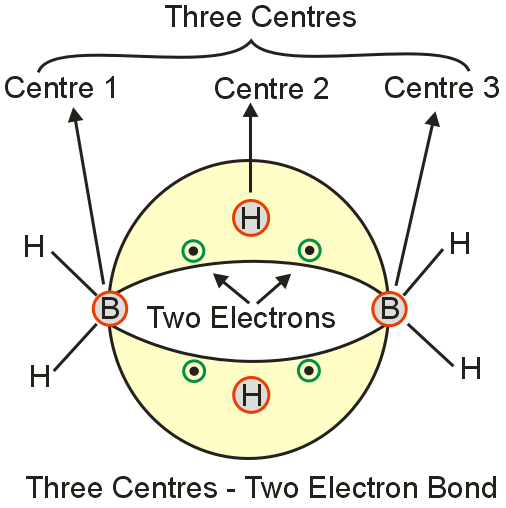
An example of molecule having a three center bond is :
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
A three-center two-electron bond is an electron deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbital s : one bonding, one non-bonding, and one anti-bonding.
The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. The simplest example of a 3c – 2e bond is in the B2H6. Hence correct answer is option D.