Engineering
Chemistry
Enthalpy of Neutralisation and Resonance Energy
Question
Calculate enthalpy change (in kJ) when 2 moles of liquid acetic acid undergoes dissociation into CH4(g) and CO2(g) from the following data.
ΔHvap [CH3COOH](ℓ) = 50 kJ/mol.
Resonance energy of CH3COOH(g) = – 50 kJ/mol
Resonance energy of CO2(g) = – 25 kJ/mol
Bonding energy (kJ/mol) :
C–H = 400; C – O = 350; O = O = 500
C–C = 350; O – H = 450; C = O = 800; H – H = 400
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
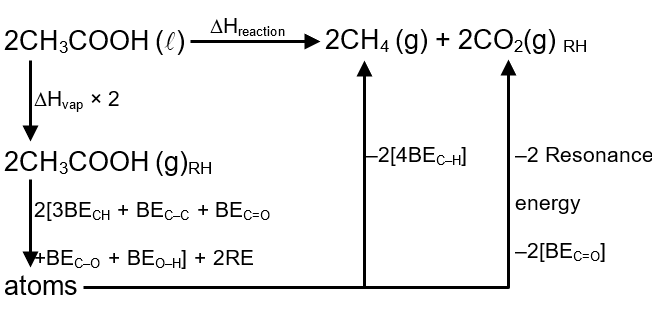
ΔH = 2 × 50 + 2 × 50 + 2 [3 × 400 + 350 + 800 + 350 + 450]
– 2 [4×400] – 2 × 25 – 4 × 800
ΔH = 50 kJ