Engineering
Chemistry
Compound of Carbon Family New
Question
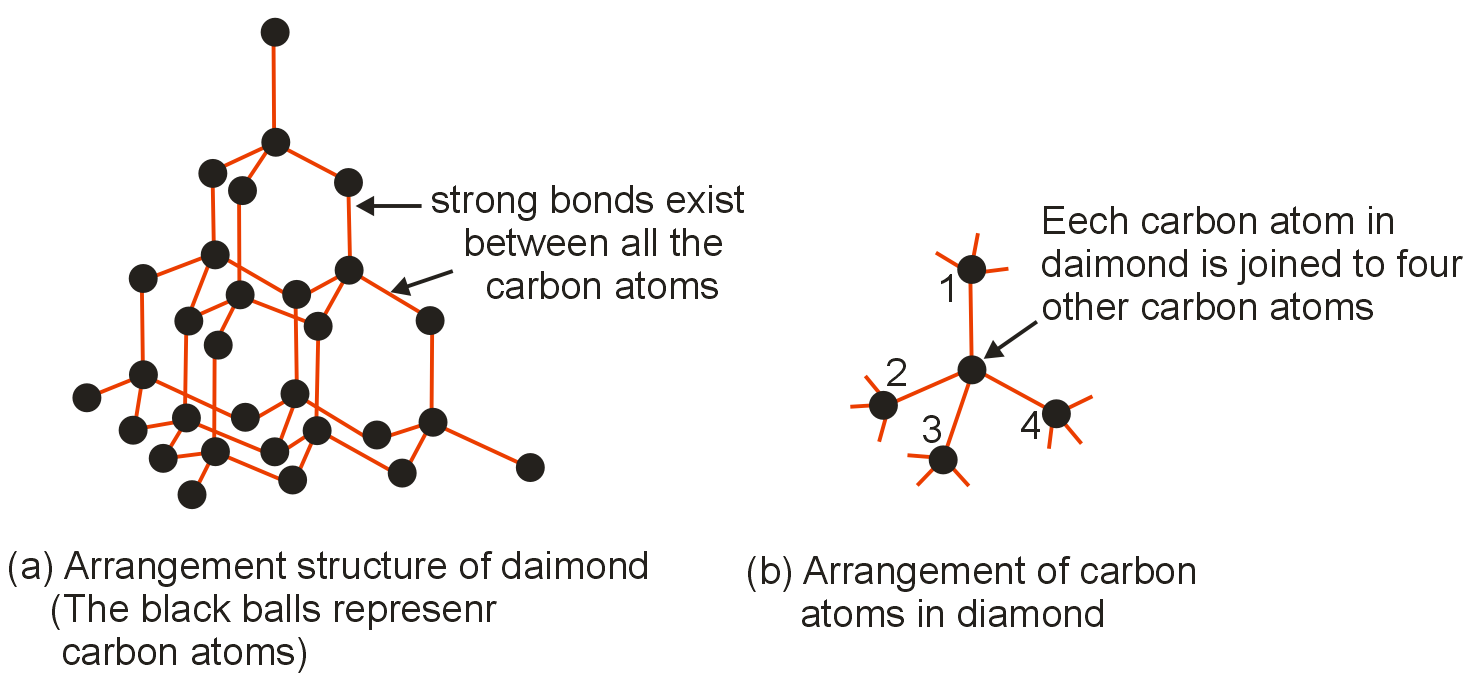
Carbon atoms in diamond are bonded with each other in a configuration :
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Diamond is the hardest material known on earth. Each carbon atom is surrounded by four other carbon atoms, which lie at the corner of a regular tetrahedron. Valence bond connects each carbon atom with four others. Carbon atoms in diamond are sp3 hybridized.
Hence the correct option is (D).