Engineering
Chemistry
Calculation of Various Parameters at Equilibrium
Le Chateliers Principle
Ammonia
Question
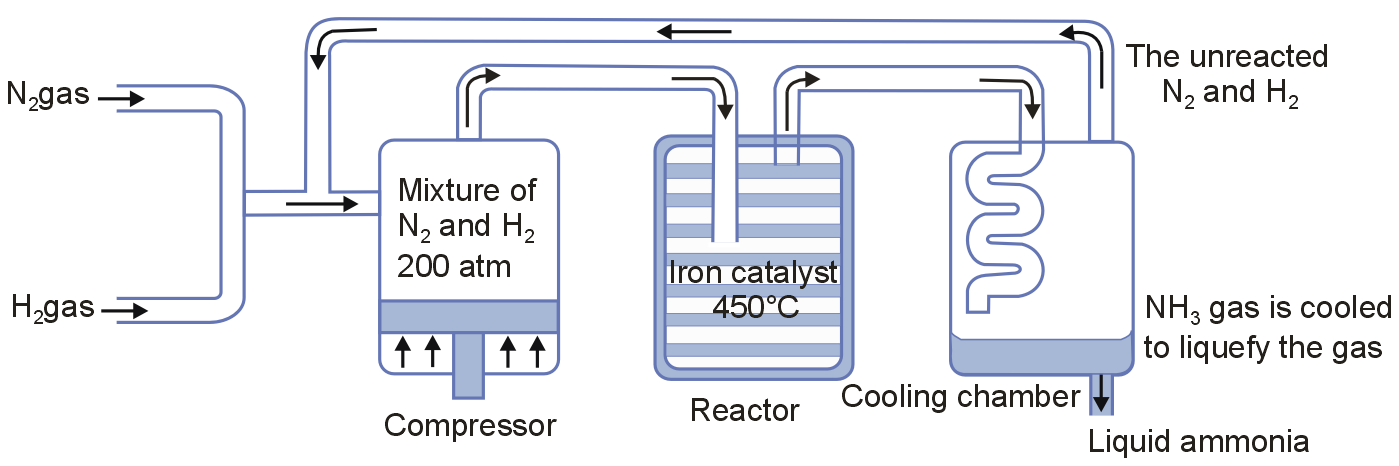
Describe industrial manufacture of ammonia by Haber's process with labelled diagram.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Large scale production of ammonia is carried by Haber's process as :
N2(g) + 3H2(g) ⇌ 2NH3(g) ΔHf = 46.1 kJ/mol
In the process, the two gases (N2 and H2) are mixed in a molar ratio of 1 : 3 and then compressed to the pressure of 200 atm. The mixture then reacts on Iron oxide catalyst (highly porous and finely divide) containing small amounts of promoters (molybdenum or alumina (Al2O3) and potassium oxide (K2O). The reaction is carried out at a high temperature of about 700 K. The ammonia gas is obtained which is then cooled to liquid ammonia and the unreacted molecules of N2 and H2 are directed back to the compression chamber.