Engineering
Chemistry
Electrolysis
Faraday Law
Question
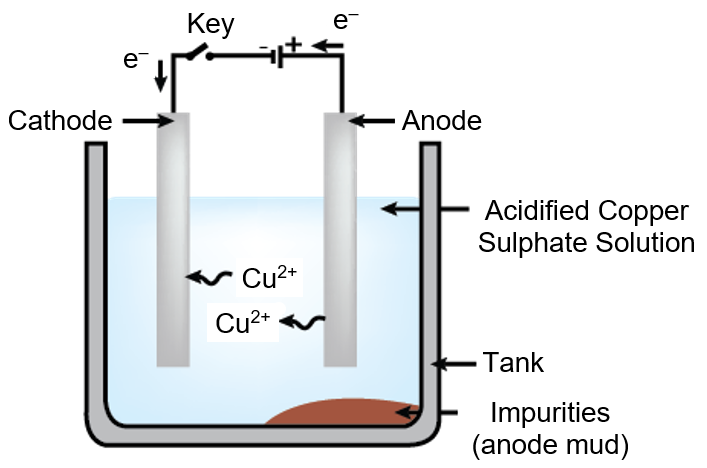
Draw the diagram of the Apparatus used in electroplating and label the following parts:
The substance to be electroplated.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
The substance to be electroplated is Copper.
Anode - Impure Copper metal rod
Cathode - Pure Copper metal rod
Electrolyte - Acidified copper sulphate solution
Process of electroplating - On the application of high voltage the pure copper metal ions from the impure copper metal rod i.e. anode dissolves into the electrolyte and the same amount of Cu2+ ion gets deposited over the cathode rod as shown in the diagram.