Engineering
Chemistry
Electrolysis
Faraday Law
Extraction of Iron and Copper
Question
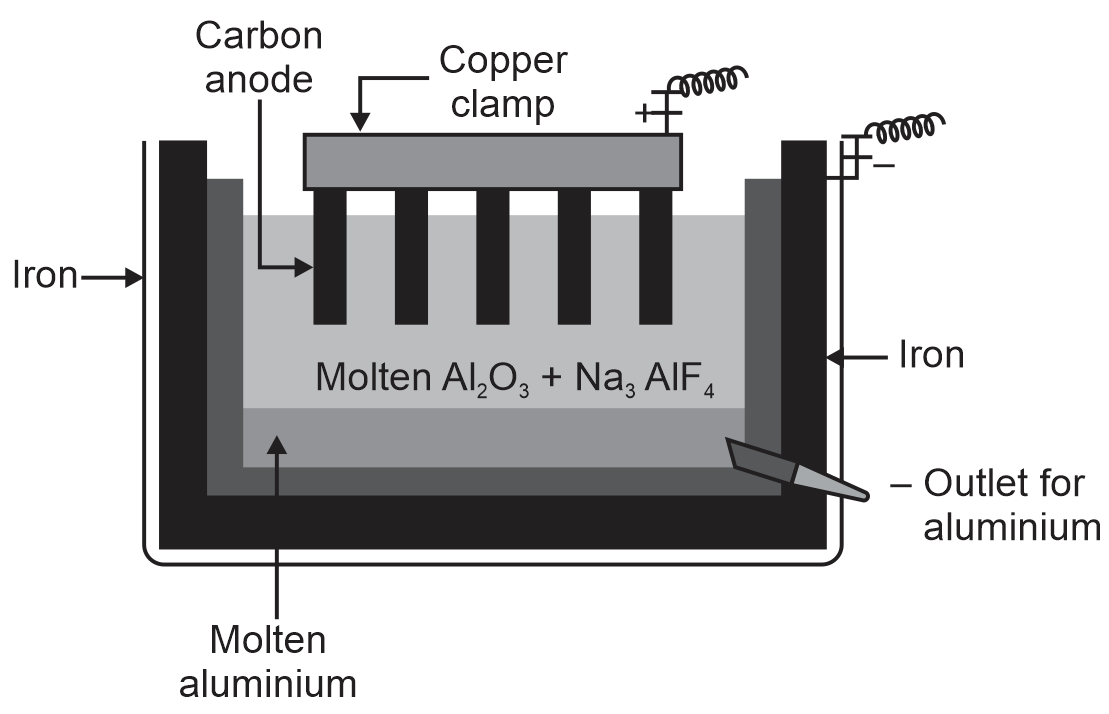
During the extraction of aluminium by Hall-He'roult process,
(i) Write a neat labeled diagram of the electrolytic cell.
(ii) Write over all cell reaction.
(iii) At which electrode oxygen gas is liberated?
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
During the extraction of aluminium by Hall-He'roult process,
(i) Neat labelled diagram of electrolytic cell is as shown.
(ii) Over all cell reaction is .
(iii) At anode oxygen gas is liberated.
C + O2– → CO + 2e–
C + 2O2– → CO2 + 4e–
At cathode, Al is obtained.
Al3+ + 3e– → Al
C + 2O2– → CO2 + 4e–
At cathode, Al is obtained.
Al3+ + 3e– → Al