Engineering
Chemistry
Henry Law and Solubility
Ideal Solution
Vander Waal Forces and Weak Forces
Question
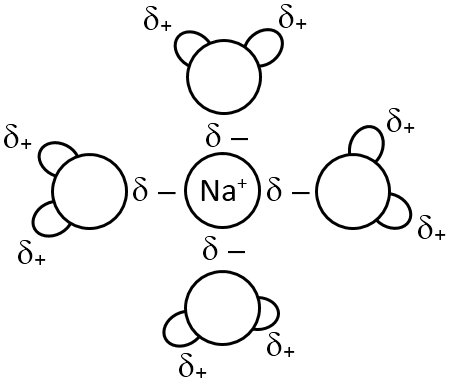
Explain ion-dipole interaction with the help of suitable example.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Ion-dipole force are generated between polar water molecules and a sodium ion. The oxygenation in the water molecule has a slight negative charge and is attracted to the position sodium ion. These intermolecular ion-dipole forces are weaker than covalent o rconis bonds