Engineering
Physics
Thermodynamics
Question
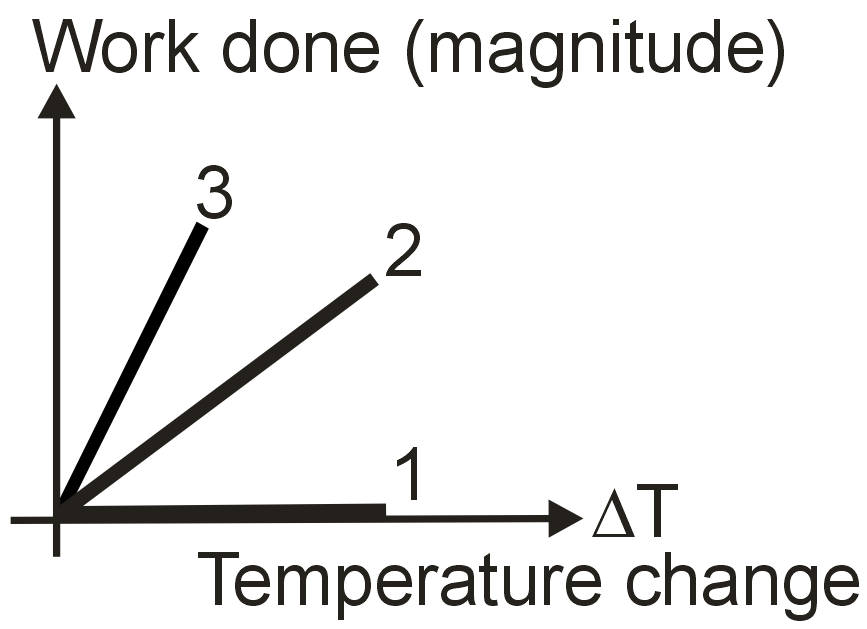
For an ideal gas graph is shown for three processes. Processes 1, 2 and 3 are respectively.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Isochoric process dv = 0
W = 0
Isobaric : W = PΔV = nRΔT
Adiabatic : W =
⇒ 0 < r – 1 < 1