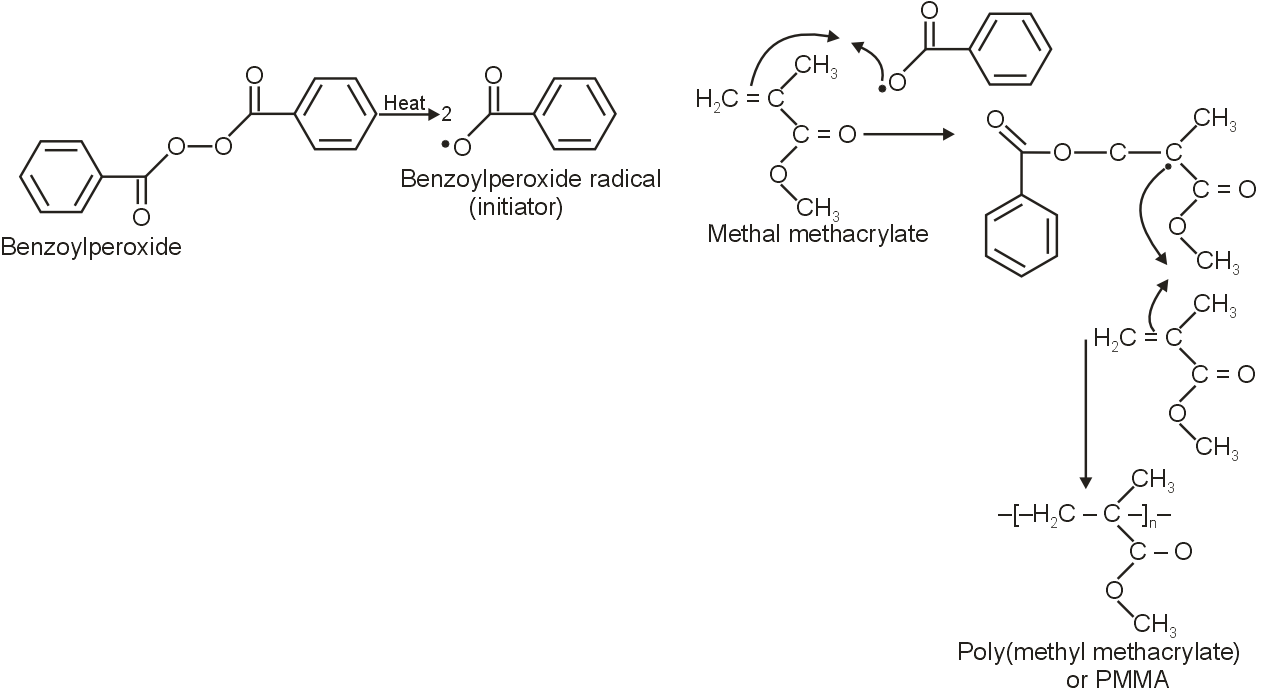
Free radical polymerisation requires a free radical initiator. The most commonly used free radical initiator is :
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
In a free radical addition polymerization, the choice of polymerization initiator depends mainly on two factors :
(a) Solubility and (b) Decomposition temperature.
If the polymerization is performed in an organic solvent, then the initiator should be soluble in that solvent and the decomposition temperature of the initiator must be at or below the boiling point of the solvent.
Commonly, Benzoyl peroxide suit these requirements.
For e.g., Benzoyl peroxide initiates the methacrylate (MMA) monomer to form a radical that can attack the double bound of another MMA monomer, to form PMMA.