Engineering
Chemistry
Alkene
Polymers
Homologous series
Question
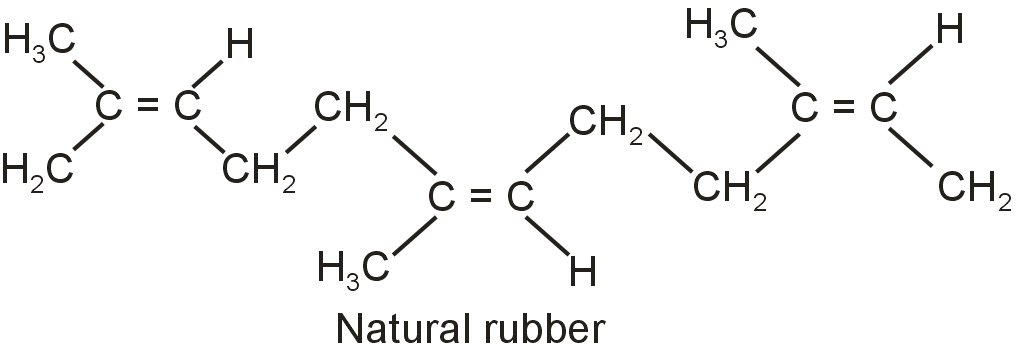
How does the presence of double bonds in rubber molecules influence their structure and reactivity ?
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Natural rubber is a linear. It is cis -1, 4 - polyisoprene. The double bonds are located between C2 and C3 of isoprene units. This cis-configuration about double bonds do not allow the chains to come closer for effective attraction due to weak intermolecular attractions. Hence, the natural rubber has a coiled structure and shows elasticity.