Engineering
Chemistry
Calculation of Various Parameters at Equilibrium
Le Chateliers Principle
Ammonia
Question
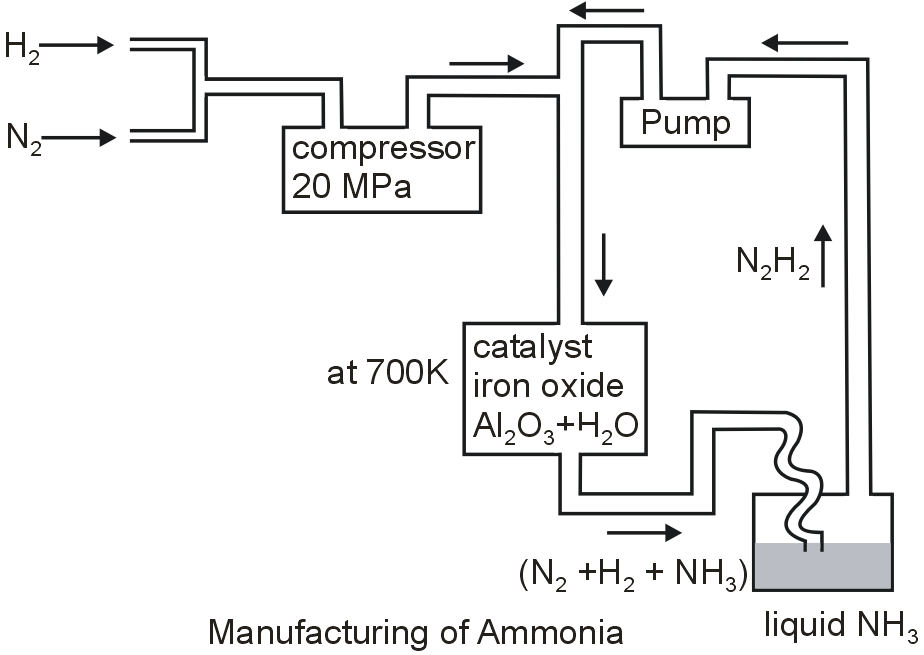
How is ammonia manufactured by Haber's process ? Explain.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
On an industrial scale, ammonia is prepared by Haber’s process. The constituents of ammonia N2 and H2 combine in a ratio of 1:3.
N2 + 3H2 ⇌ 2NH3
The reaction proceeds in the forward direction with a remarkable decrease in volume and thus the reaction is exothermic. In accordance with Le Chatelier’s principle, high pressure would favour the formation of ammonia. The optimum conditions for the production of ammonia are a pressure of approx 200 atm, a temperature of approx 700 K and the use of a catalyst such as iron oxide with small amounts of K2O and Al2O3 to increase the rate of attainment of equilibrium.