Engineering
Chemistry
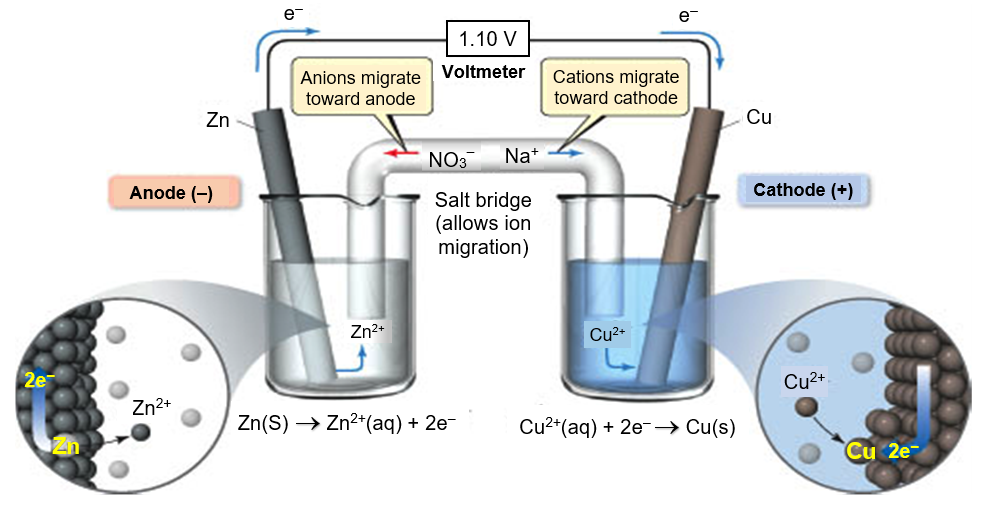
EMF Calculation and Galvanic Cell
Nernst Equation
Question
In a galvanic cell electron flow will be from:
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Electron flows opposite to the direction of current,
i.e. from low to high voltage.
∴ Electron flows from negative to the positive electrode.
Hence, the correct option is A