Engineering
Chemistry
Arrhenius Equation
First Law and Various Process
Enthalpy of Neutralisation and Resonance Energy
Question
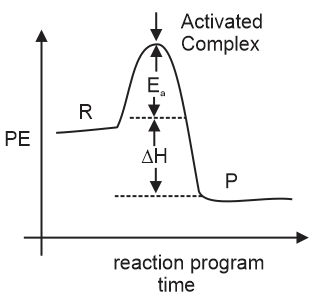
In gaseous reaction important for the understanding of the upper atmosphere H2O and O react bimolecularly to form two OH radicals. ΔH for this reaction is 72 kJ at 500 K and Ea is 77 kJ mol−1 Ea for the bimolecular recombination of two OH radicals to form H2O and O is: (in kJ mol−1)
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
The reaction is:
H2O + O → 2OH,
ΔH = −72kJ;
Ea = 77kJ/mol
2OH → H2O + O , Ea = ? (Refer to Image)
Ea for the reverse reaction
= (Ea)forward + ΔH =77 + (−72) = 5kJ