Engineering
Chemistry
Electrolysis
Faraday Law
Question
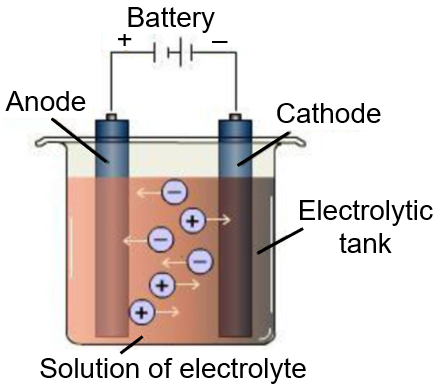
In the electrolytic cell, flow of electrons is from:
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Hint: Electrons flow from the negative to a positive end.
Step 1: A cell in which Electrical energy is used to cause a non-spontaneous oxidation and reduction reaction is called an Electrolytic cell. The electrolytic cell requires an energy input to proceed.
Step 2: Electrolytic cell contains three parts Cathode, Anode, and electrolyte.
- Positive electrode is called anode.
- Negative electrode is called Cathode.
- An electrolyte is a substance in an aqueous or molten form that dissociates into ions and conducts electricity.
- In a electrolytic cell, electrons are transferred from cathode to anode through internal supply.