Engineering
Chemistry
Equilibrium Constant and Mass Action Law
Le Chateliers Principle
Catalysis
Question
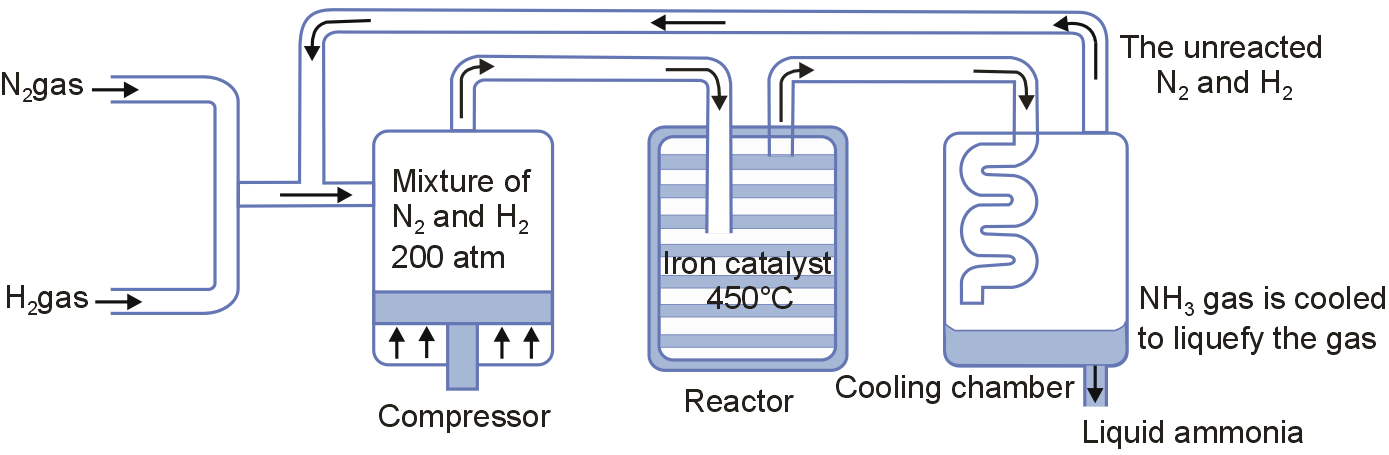
In the manufacture of ammonia by Haber's process, write the flow chart and chemical equations with optimum conditions.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
In the manufacture of ammonia by Haber's process, the flow chart is as shown.
The chemical equation is N2 + 3H2 ⇌ 2NH3
The optimum conditions are temperature = 700 K and Pressure = 200 atm.