Quinhydrone is a sparingly soluble one-to-one addition compound formed from hydroquinone and quinone. When solid quinhydrone is dissolved in an aqueous medium, equal concentrations of hydroquinone and quinone result. If a platinum wire is dippled into the solution, the potential of the electrode is govern by the reversible reaction:
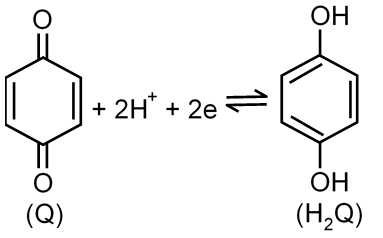
Where Q denotes quinone and H2Q denotes hydroquinone. The E° value of this half cell reaction is +0.46 V with respect to the saturated calomel reference electrode (SCE). Because the potential of the quinone-hydroquinone half reaction depends on the concentration of hydrogen ion, it is possible to use this system to measure pH. A sample solution of unknown pH was saturated with quinhydrone and a platinum electrode was dipped into it. If the potential of the such electrode was found to be +0.22 V with respect to SCE, what was the pH of the sample solution?
[Given : ]
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
E = E° – 0.06 pH
0.22 = 0.46 – 0.06 pH
0.06 pH = 0.46 – 0.22
Please subscribe our Youtube channel to unlock this solution.