Engineering
Chemistry
Simple Face and Body Centered Unit Cells
Arrangement of Constituents Density and Efficiency
Question
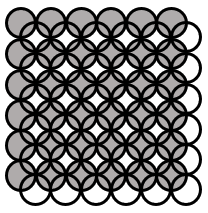
The crystal of a solid is square packing of identical spheres in each layer and spheres of one layer are placed just above the voids made by spheres in previous layer. The packing efficiency of such crystal (in %) is
[ Take: π = 3.15, ]
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
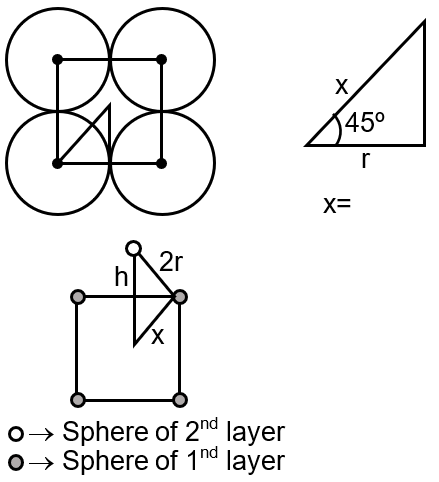
(2r)2 = h2 + x2
h2 = 2r2
height = ,
area = 4r2
Volume = area × height
= 4r2 × ,
Z = 1,
P.F. = = 0.75 ⇒ 75 %
OR
As this packing also give rise to FCC lattice \ it can be directly commented that P.F. = 0.75