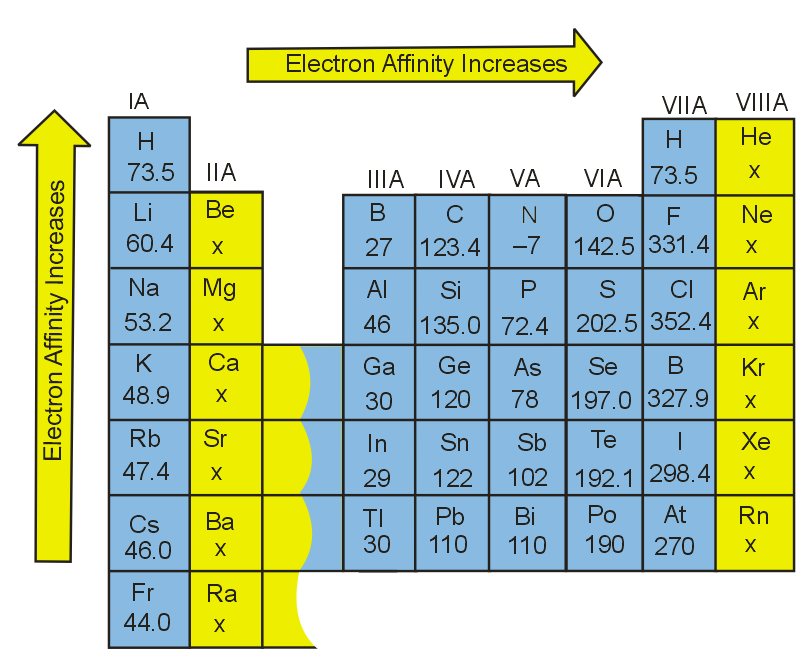
The electronic configuration of the element with maximum electron affinity is :
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Fluorine is a small atom with a small amount of space available in its 2p orbital. Because of this, any new electron trying to attach to fluorine experiences lower electron affinity from the electrons already living in the element's 2p orbital. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Therefore, chlorine has a higher electron affinity than fluorine, and this orbital structure causes it to have the highest electron affinity of all of the elements.