Engineering
Chemistry
Hybridisation
Question
The hybridisation and geometry of BrF3 molecules are :
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Steric no. of
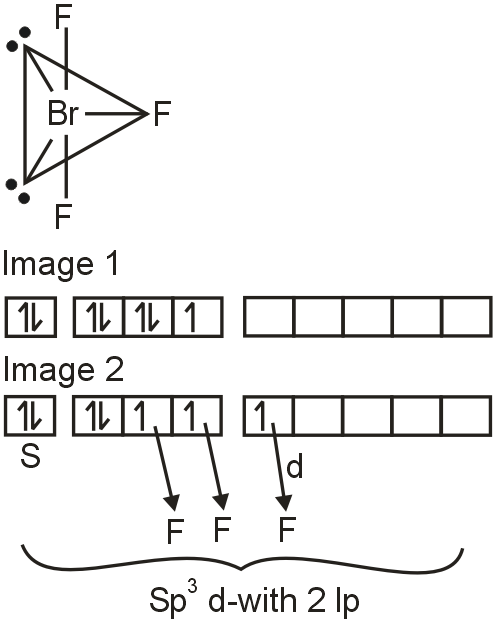
Molecular geometry is trigonal bipyramidal.
Shape is T – shaped (Refer IMAGE 01)
Br at ground state (Refer IMAGE 02)
Br – 4s24p5
Br at excited state (Refer IMAGE 03)
Br – sp3d with 2lp.