Engineering
Chemistry
Hydrogen Bonding
Hydrogen Peroxide Properties and Preparation
Question
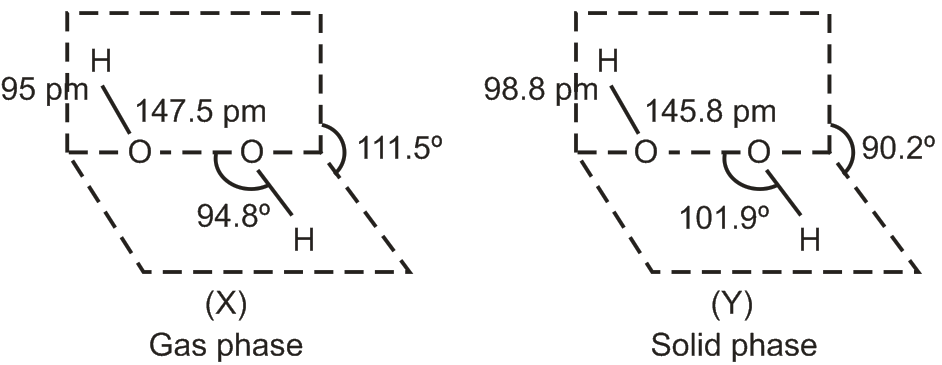
Two structure of H2O2 are drawn below. Identify the phase (X) and (Y) of H2O2.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
(X) is the structure of H2O2 in the gas phase and (Y) in the solid phase.
O – O single bond length is higher in the gas phase. H – O – O bond angle is higher in the solid phase.
Hence, the correct answer is option A.