Engineering
Chemistry
Polymers
Classification of organic compounds
Basic concepts of organic chemistry
Question
What is the utility of vulcanisation of rubber? How is vulcanisation carried out ?
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
Natural rubber has the following drawbacks :
(1) It is soft and sticky at room temperature. At high temperatures, (> 335 K), softness increases.
At low temperatures, (< 283 K), it is brittle.
Hence, to maintain elasticity, natural rubber can be used in the temperature range of 283 K – 335 K.
(2) It can absorb large amounts of water.
(3) It's tensile strength is low and it has low resistance to abrasion.
(4) It is soluble in non-polar solvents.
(5) It is easily attacked by oxidizing agents.
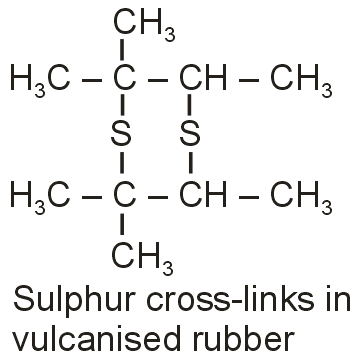
Vulcanization is carried out to improve this property. This process involves heating a mixture of raw rubber with sulphur and other additives at a temperature range between 100°C to 145°C.
After vulcanization sulphur forms cross-links at the reactive sites of double bonds and thus the rubber gets stiffened.