Engineering
Chemistry
Entropy Calculations
Gibbs Free Energy Calculations and Third Law
Intensive Extensive State and Path Function
Question
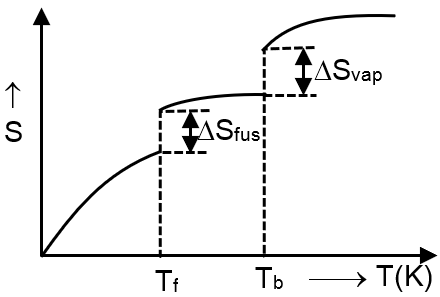
Which of the following graphs best illustrates the variation of entropy of a substance with temp.
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
At phase transitions, entropy increases at constant temperature and it is zero at absolute zero.