Engineering
Chemistry
pH Scale and Relative Strength of Acids
pH Calculation for Acids and Bases
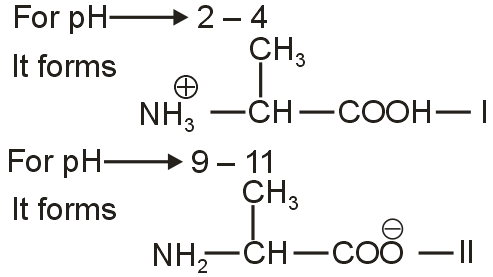
Amino acids
Question
Within the list shown below, the correct pair of structures of alanine in
(I)
(II)
(III)
(IV)
Know your College Admission Chances Based on your Rank/Percentile, Category and Home State.
Get your JEE Main Personalised Report with Top Predicted Colleges in JoSA
Solution
As the isoelectric point of Alanine is 6.1.
AtpH p
At
Option (A) is correct.
At
At
Option (A) is correct.